Can an Mri Scan Detect Inner Ear Problems?
Abstract
Intravenous gadolinium-enhanced inner-ear magnetic resonance imaging (Iv-Gd inner-ear MRI) has been used to visualize endolymphatic hydrops (EH) in clinical diagnosis of Ménière's disease (Md). All the same, lack of histological validation has led to several concerns regarding how best to interpret the resulting images. Hither, we compared hydropic changes in temporal bone specimens with the results of 4-Gd inner-ear MRI in patients with Medico. Histopathologic images of temporal bones from 37 patients with MD and x healthy controls were collected from the National Temporal Bone Bank of the Massachusetts Heart and Ear Infirmary in the U.s.. The EH ratios in the vestibule and cochlea were calculated from temporal bones using the methods used for IV-Gd inner-ear MRI, and the caste to which the saccular and utricular hydrops contributed to vestibular hydrops was measured. The presence of hydropic change in each semicircular canal was assessed using temporal bone images and compared with IV-Gd inner-ear MRI scans of 74 patients with MD. Based on man temporal bone imagery, the EH ratios in the cochlea and the vestibule on the affected side were 0.314 and 0.757, respectively. In the healthy control group, the ratio was 0.064 for the cochlea and 0.289 for the entrance hall; these values were significantly different from those for the affected side of Physician patients. The values for the affected ear were similar to the ratios from the IV-Gd inner-ear MRI scans in Doctor patients. In the lobby, saccular hydrops were more common than utricular hydrops. The average EH ratios in the saccule and utricle were 0.513 and 0.242, respectively. No significant hydropic alter from each of 3 semicircular canals was axiomatic in temporal bone histopathology. However, herniation of otolithic organs (saccule or utricle) into the lateral semicircular canal was found in 44.4% of the patients, with saccular herniation (24.viii%) more common than utricular herniation (sixteen.vii%). Although 4-Gd inner-ear MRI might not reverberate fully the results of bodily histopathology due to the limited resolution of MRI and image-processing techniques, the measured EH ratios from temporal bone specimens and Iv-Gd inner-ear MRI scans were similar. Hydropic alter in the three semicircular canals was not meaning at either the ampullated or nonampullated end. Canal invasion of vestibular hydrops seen on MRI likewise appeared in temporal bone histopathology, and saccular invasion was dominant.
Introduction
In 1861, Prosper Ménière described fluctuations in hearing loss and episodic vertigo as prove of dysfunction of the inner labyrinth rather than a central neurogenic disorder1. In 1938, Yamakawa2 and Hallpike and Cairns3 identified endolymphatic hydrops (EH) as a histopathological marker in patients with Ménière'south disease (Doc)4,5,6.
The diagnosis of MD is based on criteria proposed by the American Clan of Otolaryngology–Head and Neck Surgery7 and the Bárány Ordereight. These diagnostic criteria were based on clinical manifestations because of a lack of clear and objective measures to confirm MD.
Recently, magnetic resonance imaging (MRI) has been reported to exist a useful tool for diagnosing Medico in nigh patients through in vivo visualization of EH9. Information technology also can be used to differentiate Physician from other diseases10,11. Correlations betwixt audiovestibular tests and hydrops level have been reported12,thirteen. Pure-tone audiometry and electrocochleography (ECoG) have been correlated with severity of hydrops in the cochlea and vestibulefourteen. In dissimilarity, the correlation of caloric part and vestibular evoked myogenic potential (VEMP) with hydrops level is controversial15. In addition, changes in hydrops level later on treatment or over time have been reportedxvi. The successful utilise of MRI to visualize EH and assess the hydrops level in the inner ear would provide valuable information regarding diagnosis, treatment options, and treatment outcomes in patients with MD. Optimal methods to obtain and validate reliable data are required.
Notwithstanding, because image acquisition and processing and data interpretation differ among study groups, concerns regarding the technique'southward accurateness persist. Image quality can differ according to the delivery method for gadolinium (intravenous vs. intratympanic) and MRI parameters such as inversion time17. In improver, the method used to measure hydrops level might not be authentic. MRI data must be validated past comparisons with histopathologic findings from the temporal basic of patients with Medico.
In previous studies, we reported that intravenous gadolinium-enhanced inner-ear MRI (IV-Gd inner-ear MRI) was useful for diagnosis of definite MD. Hydrops level was correlated with pure-tone thresholds, cochlear summating potential/auditory nerve action potential on ECoG, and caloric tests. However, information technology was not correlated with VEMP thresholdsxiii. We also documented that discrepancies betwixt caloric tests and video head impulse test (vHIT) results were the product of hydropic change in the horizontal canal rather than actual vestibular loss. Recently, deep-learning techniques using artificial intelligence have been used to measure automatically the hydrops ratioxviii. Advances in image acquisition and measuring techniques might be applicative. Nonetheless, several issues accept to exist clarified with respect to histologic findings.
This study was designed to identify histopathologic features required to address successfully several controversial issues raised past previous MRI studies. First, we compare our methods of measuring hydrops ratio in the cochlea and lobby. Second, nosotros accost the controversy surrounding the correlation of VEMP and MRI. Assuming different contributions from saccular and utricular hydrops in vestibular hydrops, nosotros measured saccular and utricular hydrops levels separately to assess the contributions of saccules and utricles in vestibular hydrops. Third, to confirm the being of hydrops change in each semicircular canal, the hydrops level at the ampullated and nonampullated ends of each semicircular canal was measured. Fourth, because the correlational of caloric tests and MRI has been controversial, we investigated hydropic changes and canal invasion of vestibular hydrops. Our results help to elucidate these controversial issues and provide the groundwork for further studies.
Results
EH ratios for the cochlea and anteroom in temporal bone histopathology and 4-Gd inner-ear MRI
Ratios for EH from temporal bone specimens in 37 Doc patients and 10 healthy control patients were measured in the cochlea and foyer (Fig. 1). The average EH ratio [standard deviation (SD)] in the cochlea was 0.314 (0.118) on the affected side (54 ears) and 0.064 (0.022) in the healthy control group. In the antechamber, the average EH ratio was 0.757 (0.205) and 0.289 (0.062) in the affected side and good for you controls, respectively. In both cochleas and vestibules, the affected ears showed a significantly college EH ratio value (p < 0.001) compared with healthy controls.
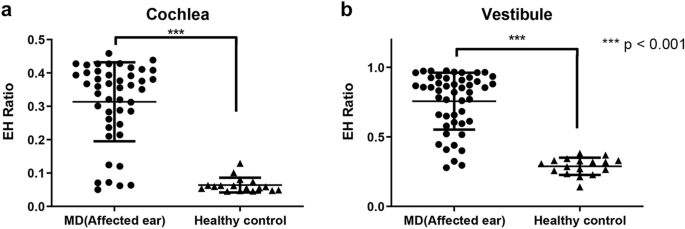
Endolymphatic hydrops (EH) ratios analyzed from histopathology images. In both cochlea (a) and vestibule (b), the EH ratio of the affected ear was significantly college (p < 0.001) than that of the healthy control grouping.
These values were compared with the EH ratio calculated from inner-ear MRI of 72 patients with unilateral definite Medico. The mean hydrops ratio in the cochlea from inner ear MRI was 0.372 (0.164), which was similar to the hateful hydrops ratio calculated from histopathology (mean, 0.314) (Fig. 2a). In addition, the mean hydrops ratio in the vestibule was not significantly different between histopathology (0.757) and MRI (0.533; SD, 0.250) (Fig. 2b). Therefore, the mean hydrops ratio in the cochlea and vestibule was comparable betwixt histopathology and inner ear MRI using our methods.
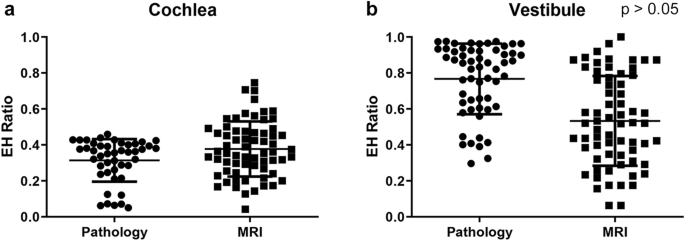
Comparison of histopathology and MRI endolymphatic hydrops (EH) ratios in affected ears. The hateful values for the EH of affected ears in the cochlea (a) and vestibule (b) were not significantly unlike betwixt histopathology and MRI.
Contributions of saccular and utricular hydrops to vestibular hydrops
From IV-Gd inner-ear MRI, the mean hydrops ratio (SD) in the affected lobby was 0.533 (0.250). However, it was difficult to divide saccular and utricular hydrops in the MR images using our method. We therefore evaluated saccular and utricular hydrops separately using temporal bone specimens. Using specimens from the affected side, the mean numbers (SD) of saccular and utricular hydrops were 0.513 (0.214) and 0.242 (0.124), respectively. The saccular hydrops ratio was significantly higher (p < 0.001) than the utricular hydrops ratio (Fig. 3).
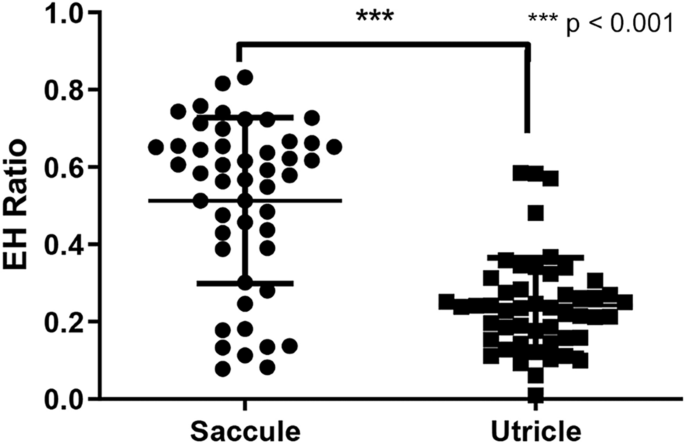
The ratio of endolymphatic hydrops (EH) measured in the saccule and utricle of the anteroom on histopathology.
EH ratios in semicircular canals in temporal bone histopathology
The EH ratios in three semicircular canals were measured at either the ampullary or non-ampullary region. For the affected ear, the hateful endolymphatic space ratios (SD) in the ampullary region of anterior, lateral, and posterior canals were 0.565 (0.096), 0.511 (0.158), and 0.518 (0.068), respectively. In the unaffected ear, the EH ratios of the anterior, lateral, and posterior canals were measured to be 0.515 (0.050), 0.456 (0.092), and 0.491 (0.080), respectively, compared with the afflicted side. Overall, no significant differences were plant between the affected, unaffected, and salubrious control groups (Fig. 4a–c). The same results were obtained from non-ampullary regions of each semicircular canal (Fig. 4d–f). We concluded that hydropic change was not significant in any semicircular culvert in Medico.
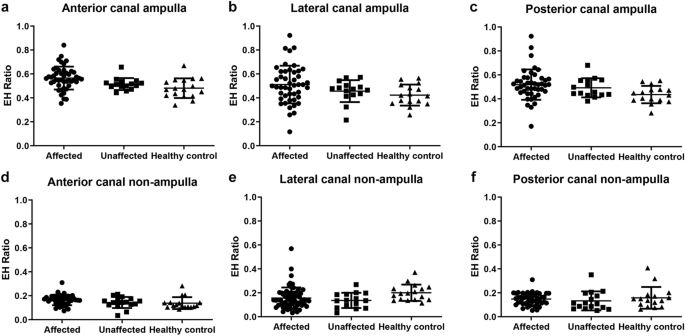
The endolymphatic hydrops (EH) ratio in all semicircular canals analyzed from histopathology images. All canals were analyzed by dividing them into an ampulla end (a–c) and a not-ampulla end (d–f). No statistical differences were axiomatic between them.
Canal extension of EH from the anteroom in temporal bone histopathology and 4-Gd inner-ear MRI
In IV-Gd inner-ear MRI, canal extension of EH from the vestibule was institute in 29 cases (40.three%), as shown in Fig. 5a. It was hard to determine in the MRI scans whether this was due to extension of the utricle or the saccule. However, in temporal bone specimens, they could be distinguished clearly, every bit shown in Fig. 5b,c (saccule and utricle, respectively). In temporal bone specimens, a canal extension from the foyer was observed in 24 (44.4%) of the 54 afflicted ears. Amongst them, saccular hydrops (n = xv) was observed more commonly to extend into the lateral semicircular canal (LSCC) compared with utricular hydrops (n = 9) (Fig. 5d). There was no example in which the saccule or utricle extended to the LSCC in the healthy control group.
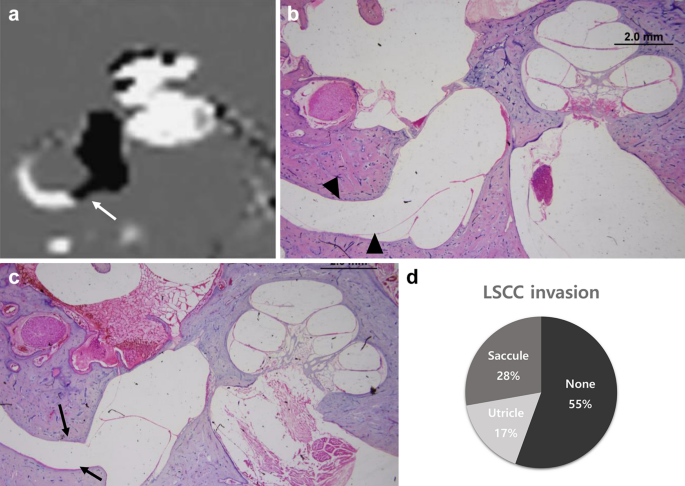
Vestibule endolymphatic hydrops (EH) observed on MRI and histopathology images. On inner-ear MRI, the hydrops of the vestibule extended toward the non-ampullary region (white pointer) of the lateral semicircular canal (LSCC) (a). In the histopathological department, the saccular hydrops (blackness arrowhead) progressed and extended to the LSCC, (b) and the utricle also invaded the LSCC (blackness arrow) (c). The charge per unit of extension of vestibular hydrops to the LSCC in histopathological images of MD patients (d, 72 ears). *(a) image was generated by OsiriX Md epitome software(version seven.5.1 64-scrap, https://www.osirix-viewer.com).
Discussion
This study was intended to validate data from IV-Gd inner-ear MRI visualizations of the EH of the cochlea and lobby by comparison them with histopathologic findings in human being temporal bone specimens. Hydropic changes in the endolymphatic space of the semicircular canal and culvert invasion of vestibular hydrops were investigated through histopathologic review.
Because EH has been known every bit a histopathological marker since the 1930s19,twenty, histopathologic assay has long been applied to MD patients. Nevertheless, the pathologic mechanisms underlying the development of EH are not clear21. Gadolinium-enhanced inner-ear MRI was developed to visualize endolymphatic hydropic alter in the inner ears of patients with MD, and multiple lines of research are possible regarding the pathogenesis of MD and other inner-ear diseases related to hydropic change22.
Methods for visualizing EH by inner ear MRI include calculating the endolymphatic hydrops ratio or the ratio of saccule to utricle. The method used to measure hydrops ratio in the vestibule was introduced by Naganawa et al.23, and it could not assess easily the contributions of saccule and utricle in vestibular hydrops24. In contrast, the saccule to utricle ratio inversion (SURI) method can be useful for measuring saccular hydrops in early hydrops; even so, it is difficult to calculate the saccule to utricle ratio in severe hydrops25,26.
Two delivery methods for Gd have been proposed: intratympanic and intravenous. Each method presents advantages and disadvantages, including patient compliance, waiting time, and perilymph signal-to-dissonance ratio. In improver, because MRI conquering techniques and qualitative and quantitative methods for analyzing hydrops differ among research teams, the resulting information are inconsistent. Nosotros therefore needed to validate MRI data past comparing them with histopathologic findings from temporal bone specimens of Md patients. Both IV-Gd inner-ear MRI and quantitative analysis equally described by Nagakawa et al.23 were used for this written report.
Comparing of hydrops ratio in the cochlea and entrance hall in temporal bone specimens and 4-Gd inner-ear MRI
Inner-ear MRI is used widely to visualize EH in the cochlea and foyer of patients with definite MD. Between xc and 100% of MD patients reportedly exhibit prominent EH in the cochlea and/or vestibule according to MRI scans24,27,28. This frequency is like to that associated with histopathological studies29. In the histopathology specimens used in the present study, the EH ratio of the affected side averaged 0.314 for the cochlea and 0.757 for the lobby. This ratio is like to that observed in actual IV-Gd inner-ear MRI (cochlea, 0.372; vestibule, 0.533). Although perfect comparisons are incommunicable because the subjects of the MRI and histopathology specimens were different, the hydrops ratio obtained from the IV-Gd inner-ear MRI was comparable to that of real temporal bone specimens. As a event of MRI analysis, some of the EH ratios were less than 0.2, but this was a result of the presence of hydrops in only the cochlea or vestibule alone, non both. In all affected ears, the EH ratio of either cochlea or foyer exceeded 0.3 in MRI, and results of histopathological reviews were like.
Role of saccular and utricular hydrops in EH in the antechamber: implications for VEMP correlation
Although MRI techniques have advanced in contempo years, accurate evaluation of EH remains elusive. For example, in the case of severe hydrops shown in Fig. 5a, it is difficult to distinguish between the saccule and utricle in the vestibule using our method. The correlation between VEMP and vestibular hydrops is controversial. Gurkov pointed out that these results were due to the inhomogeneity of diagnostic criteria, hydrops quantification, and VEMP quantification22. The cVEMP test is commonly used to appraise saccular dysfunction. Several studies have reported that the sensitivity of cVEMP in Doc patients ranges from 50 to 70%30,31,32. As our results showed, VEMP correlation might be dependent on the involvement of otolithic organs. In temporal os specimens, vestibular hydrops detected by MRI was influenced by either saccular or utricular hydrops, with saccular hydrops the more common of the ii. Therefore, VEMP results can exist affected past involvement of otolithic organs and severity.
Hydrops changes in semicircular canals and hydropic extension into semicircular canals: Implications for caloric test correlations
McGarvie et al. described a mechanism to explain the subtract in caloric response and discrepancy of vHIT results33. According to their theory, hydropic expansion of the endolymphatic duct in MD patients increases turbulence inside the duct, and this can dissipate the hydrostatic pressure caused by thermally induced density differences and diminish or eliminate deflection of the cupula. In our results, no significant dilation of semicircular canal endolymphatic ducts was observed on the affected sides in MD patients, even though there were several outliers at the ampullated end (Fig. 4). However, the saccule or utricle was enlarged and invasion into the non-ampulla end of LSCC was seen in 45% of cases (Fig. 5d). Saccular hydropic extension into the horizontal canal has been reported frequently, just utricular hydropic extension is rare in histopathological analysis34. In our analysis, utricular invasion was observed in 17% of patients, which led united states of america to speculate that the deterioration of caloric response in MD patients was due to antechamber invasion, non dilatation of the endolymphatic duct within the canal. In a previous written report using Four-Gd inner-ear MRIxiii, significant caloric response degradation (culvert paresis) was seen only in these groups and not in those without canal invasion, which supports our findings and explains the recently reported dissociation of caloric and vHIT results in patients with MD or delayed EH35,36.
Our research has several limitations. The temporal bone specimens were not from the same patients subjected to Iv-Gd inner-ear MRI, prohibiting directly histological validation. The subjects who supplied the temporal bone specimens were older and more than likely to have stop-stage Md without agile vertigo spells compared with the subjects studied by MRI who had likely agile MD. In improver, the severity of hydrops might differ between the two groups. Despite these limitations, our results showed like hydrops ratios betwixt the 2 groups, and the contributions of the saccule and utricle to vestibular hydrops and culvert invation were identified. Additionally, several controversial issues regarding VEMP and caloric result correlations were explained. In this study, we validated MRI findings of hydrops ratio and canal extension past comparing them to histopathologic findings and demonstrated controversial issues such every bit VEMP and caloric test correlation.
Conclusions
Although we did not make direct comparisons between inner ear MRI and histopathology from the same patients, our comparisons showed that hydrops ratios measured using inner ear MRI were like to those obtained through histopathology. In improver, hydropic alter in the semicircular canal was not significant at either the ampullated or not-ampullated end, and the canal typically was invaded by vestibular hydropic extension, mainly by the saccule. Due to the limited resolution of MRI and the demand for additional image processing, we were unable to fully duplicate the results of actual histopathology. However, MRI is promising for report of MD patients.
Materials and methods
Subject enrollment for MRI
Data from 72 Md patients (33 males, 39 females; mean age = 49.ix years, age range = 19–75 years) were evaluated in this study. All patients were diagnosed with definite Md according to the Committee of the Bárány Lodge diagnostic criteria8. Seventy-one patients had unilateral Md. The average symptom duration was 51.nine months (range = one.3–281.9 mo), and there were no patients with history of whatsoever other otologic disease or surgery. The pure tone average (0.5K, 1K, 2K, 4K) at the lesion side was l.nineteen dB HL (SD = 19.58 dB). Written informed consent was obtained from all participants prior to conducting the study. This study was approved by the Institutional Review Board of Samsung Medical Eye following the tenets of the Announcement of Helsinki (IRB File No. 2018-11-020).
MRI protocol
The protocol described below is the same every bit that reported past Naganawa et al. in 201237. IV-Gd inner-ear MRI was performed on a 3.0-T unit (MAGNETOM Skyra; Siemens Medical Solutions, Erlangen, Deutschland) using a 32-channel array caput coil. All patients waited iv h afterward a single dose (0.one mL/kg or 0.i mmol/kg body weight) of Four-administered gadobutrol (gadolinium-DO3A-butriol, GADOVIST 1.0; Schering, Berlin, Germany) before undergoing MRI. All patients underwent heavily T2-weighted (hT2W) magnetic resonance cisternography (MRC) for anatomical reference of total endolymphatic fluid, hT2W–3D-FLAIR with an inversion time of 2250 ms [positive perilymph paradigm (PPI)], and hT2W–3D-IR with an inversion time of 2050 ms [positive endolymph prototype (PEI)] for evaluating EH. Repetition fourth dimension was 9000 ms, repeat time was 540 ms, and voxel size was 0.5 × 0.five × i.0 mm.
The PEI parameters were the same as those for PPI, with the exception that PEI had an inversion time of 2050 ms. The MRC, PPI, and PEI employed identical fields of view, matrix sizes, and slice thicknesses to facilitate comparisons. We produced HYDROPS images on the scanner console past subtracting the PEI from the PPI. To increase the contrast-to-noise ratio of the HYDROPS images, HYDROPS-Mi2 images were generated on a DICOM viewer (OsiriX Doc prototype software, version 7.5.1 64-scrap; Pixmeo Sarl, Bernex, Switzerland, https://www.osirix-viewer.com) by multiplying the HYDROPS and MRC images23.
All patients underwent pure-tone audiography (PTA) at vi frequencies (0.25, 0.5, i.0, 2.0, iv.0, and 8.0 kHz). A semi-automated testing device was used in a sound-attenuating booth that met the prevailing standards for maximum permissible ambience noise levels during audiometry (ANSI, 1977).
Data notation from MRI
1 neuro-radiologist and one neuro-otologist independently evaluated the MRI scans. According to methods proposed by Naganawa et al.23, each physician manually drew a profile of the cochlea and antechamber on the MRC prototype. The region of interest (ROI) was established as follows. one. First, the image window level and width were altered to 400 and g pixels, respectively, to obtain optimal visual clarity. (2) For the cochlea ROI, the piece visualizing the cochlea turns (basal, middle, and apical) was selected. If every turn was visible on 2 or more slices, the slice with the greatest elevation of the modiolus was chosen equally a representative cochlea piece. (3) For the vestibular ROI, the everyman slice in which the LSCC band was visible for more than 240º was selected, and the ampulla was excluded on MRC images. The ROIs drawn on Iv-Gd inner-ear MRI were copied and pasted onto HYDROPS-Mi2 images. The histogram function in the OsiriX program was used to approximate the numbers of pixels in the ROI and with negative signal intensity values (i.eastward., endolymph) in the ROI. The EH ratio was calculated manually as the number of pixels for the endolymph in the ROI divided by the total number of pixels in the ROI.
Man temporal os specimens
Temporal os histopathologic specimens were collected from the National Temporal Bone Database in Massachusetts Eye and Ear Infirmary in the United States. A keyword search of the database using the term "Ménière" produced 105 cases (May 2019). Amongst them, patients who were diagnosed as not-Md in their lifetime and those who underwent a labyrinthectomy, decompression or shunt, cochlear implant, stapedotomy, or stapedectomy were excluded. In add-on, patients were excluded when their slides were difficult to read due to artifacts or moderate-to-severe post-mortem autolysis. In total, 37 Doctor cases (72 ears) were included in the analysis (18 males, xix females). Subsequently histologic review of 37 patients, 13 were found to have bilateral endolymphatic hydrops and were diagnosed with bilateral Md. The average of the four frequencies on the lesion side was 63.94 dB HL (SD = 22.14 dB), but accurate evaluation of symptom duration was not possible considering many records were missing. Patient history and PTA results were reviewed in the salubrious control group, and ten patients (17 ears, 4 males, 6 females) with no hearing loss and no history of vertigo were analyzed. Their boilerplate pure tone hearing was 40.44 dB HL (SD = 27.59). All specimens were prepared for light microscopy past fixation in 10% buffered formalin (or Heidenhain's Susa solution), decalcification in ethylene diamine tetra-acetic acid or trichloroacetic acrid, and aridity in graded alcohols. The samples were then embedded in celloidin and sectioned at a thickness of 20 mm through the axial aeroplane. Every tenth section was stained with hematoxylin and eosin and mounted on glass slides.
Information notation from temporal bone specimen
To measure the endolymphatic space of cochlear, anteroom, and semicircular canals, we used ImageJ software (http://rsb.info.nih.gov/ij), which is freely available and the most widely used scientific image analysis program38. Representative slices of the cochlea and vestibule were selected in the aforementioned way as for MRI analysis, and images were obtained of the representative slide magnified 200 × with an optical microscope. I neuro-otologist manually drew a contour of the cochlea and anteroom on the pathology slide image using ImageJ. The cochlea was divided into basal, middle, and apical turns, and the boundaries of the scala media (the space between the Reissner's membrane and the basilar membrane, endolymphatic space) of each plough were drawn. After adding this endolymphatic infinite surface area, the EH ratio was obtained by dividing information technology by the whole cochlea area. The software too draws the contours of utricle and saccule from the representative image slide and divides them into the entire antechamber surface area (Fig. 6). The semicircular canals were measured past dividing the culvert's cross-sectional area by the surface area of the endolymphatic space in each of the ampullary and non-ampullary regions.
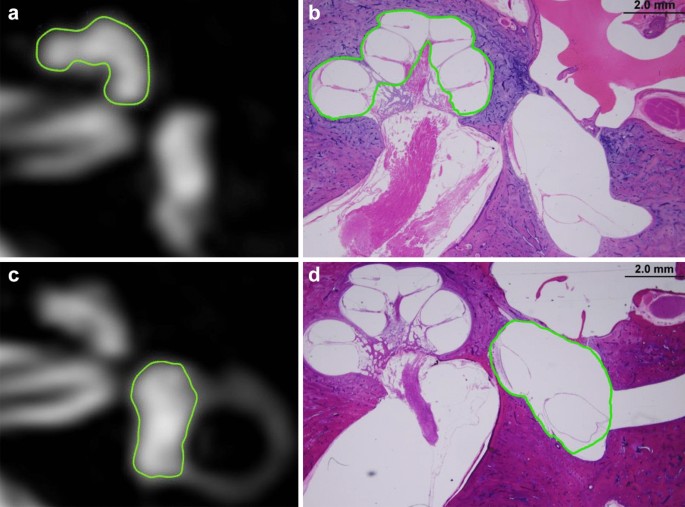
In the MRC image of the inner ear MRI and histopathology image, the region of interest for each organ is indicated by the cochlea (a,b) and lobby (c,d), respectively. *(a) and (c) image was generated by OsiriX Dr. prototype software (version vii.five.1 64-bit, https://www.osirix-viewer.com).
Statistical analysis
Nosotros used SPSS eighteen.0 software (SPSS Inc., Chicago, IL, USA) for all statistical analyses and adopted a p-value < 0.05 equally the statistical significance threshold. To compare the average of the EH ratios between groups, an independent sample t test or Mann–Whitney test was performed after the normality test.
References
-
Baloh, R. W. Prosper Meniere and his disease. Arch. Neurol. 58, 1151–1156 (2001).
-
Yamakawa, Yard. Hearing organ of a patient who showed Meniere's symptoms. J. Otolaryngol. Soc. Jpn. 44, 2310–2312 (1938).
-
Hallpike, C. South. & Cairns, H. Observations on the pathology of Meniere's syndrome. J. Laryngol. Otol. Neurotol. 53, 625–655 (1938).
-
Klis, J. F. & Smoorenburg, Grand. F. Cochlear potentials and their modulation by low-frequency audio in early endolymphatic hydrops. Hear. Res. 32, 175–184 (1988).
-
Schuknecht, H. F. In Meniere'due south Disease (ed. Harris, J. P.) 41–52 (Kugler Publications, 1999).
-
Okuno, T. & Sando, I. Localization, frequency, and severity of endolymphatic hydrops and the pathology of the labyrinthine membrane in Meniere'south disease. Ann. Otol. Rhinol. Laryngol. 96, 438–445 (1987).
-
Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere'south affliction. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngol. Head Neck Surg. 113, 181–185 (1995).
-
Lopez-Escamez, J. A. et al. Diagnostic criteria for Meniere'due south disease. J. Vestib. Res. 25, 1–7 (2015).
-
Nakashima, T. et al. Grading of endolymphatic hydrops using magnetic resonance imaging. Acta Otolaryngol. Suppl. 129, five–viii (2009).
-
Inui, H., Sakamoto, T., Ito, T. & Kitahara, T. Magnetic resonance-based volumetric measurement of the endolymphatic space in patients with Meniere's disease and other endolymphatic hydrops-related diseases. Auris Nasus Larynx 46, 493–497 (2019).
-
Attye, A. et al. Endolymphatic hydrops imaging: Differential diagnosis in patients with Meniere illness symptoms. Diagn. Interv. Imaging 98, 699–706 (2017).
-
Gurkov, R., Flatz, W., Louza, J., Strupp, Grand. & Krause, E. In vivo visualization of endolyphatic hydrops in patients with Meniere'due south illness: Correlation with audiovestibular role. Eur. Arch. Otorhinolaryngol. 268, 1743–1748 (2011).
-
Cho, Y. Southward. et al. Usefulness of intravenous gadolinium inner ear MR imaging in diagnosis of Meniere'due south disease. Sci. Rep. 8, 17562 (2018).
-
Seo, Y. J., Kim, J., Choi, J. Y. & Lee, W. S. Visualization of endolymphatic hydrops and correlation with audio-vestibular functional testing in patients with definite Meniere'south disease. Auris Nasus Larynx twoscore, 167–172 (2013).
-
Andrade, I. V., Santos-Perez, Southward., Diz, P. K., Caballero, T. Fifty. & Soto-Varela, A. Correlation betwixt bithermal caloric test results and vestibular evoked myogenic potentials (VEMPs) in normal subjects. Eur. Arch. Otorhinolaryngol. 270, 1623–1628 (2013).
-
Fiorino, F., Pizzini, F. B., Beltramello, A. & Barbieri, F. Progression of endolymphatic hydrops in Meniere's affliction as evaluated by magnetic resonance imaging. Otol. Neurotol. 32, 1152–1157 (2011).
-
Eliezer, Yard., Gillibert, A., Tropres, I., Krainik, A. & Attye, A. Influence of inversion time on endolymphatic hydrops evaluation in 3D-FLAIR imaging. J. Neuroradiol. 44, 339–343 (2017).
-
Cho, Y. Due south. et al. Automated measurement of hydrops ratio from MRI in patients with Meniere's disease using CNN-based segmentation. Sci. Rep. 10, 7003 (2020).
-
Gussen, R. Meniere syndrome. Compensatory collateral venous drainage with endolymphatic sac fibrosis. Curvation. Otolaryngol. 99, 414–418 (1974).
-
Yazawa, Y. & Kitahara, Yard. Electron microscopic studies of the endolymphatic sac in Meniere's disease. ORL J. Otorhinolaryngol. Relat. Spec. 43, 121–130 (1981).
-
Wackym, P. A. Histopathologic findings in Meniere's affliction. Otolaryngol. Caput Cervix Surg. 112, 90–100 (1995).
-
Gurkov, R. & Louza, J. Hydropic ear disease: Structure–office correlations and local depression-dose contrast awarding. Otol. Neurotol. xl, 692–693 (2019).
-
Naganawa, S. et al. Semi-quantification of endolymphatic size on MR imaging after intravenous injection of single-dose gadodiamide: Comparison between two types of processing strategies. Magn. Reson. Med. Sci. 12, 261–269 (2013).
-
Nakashima, T. et al. Endolymphatic hydrops revealed past intravenous gadolinium injection in patients with Meniere's disease. Acta Otolaryngol. 130, 338–343 (2010).
-
Lopez-Escamez, J. A. & Attyé, A. Systematic review of magnetic resonance imaging for diagnosis of Meniere illness. J. Vestib. Res. 29, 121–129 (2019).
-
Attyé, A. & Eliezer, 1000. MRI identification of the saccule? Do it yourself!. Otol. Neurotol. 39, 1070–1071 (2018).
-
Pyykko, I., Nakashima, T., Yoshida, T., Zou, J. & Naganawa, S. Meniere's disease: A reappraisal supported by a variable latency of symptoms and the MRI visualisation of endolymphatic hydrops. BMJ Open up 3, e001555 (2013).
-
Fiorino, F., Pizzini, F. B., Beltramello, A., Mattellini, B. & Barbieri, F. Reliability of magnetic resonance imaging performed afterward intratympanic administration of gadolinium in the identification of endolymphatic hydrops in patients with Meniere's disease. Otol. Neurotol. 32, 472–477 (2011).
-
Loureiro, R. Thousand. et al. The role of magnetic resonance imaging in Meniere disease: The current land of endolymphatic hydrops evaluation. Einstein (Sao Paulo) 17, eMD4743 (2019).
-
Rauch, S. D., Zhou, Thou., Kujawa, S. One thousand., Guinan, J. J. & Herrmann, B. Due south. Vestibular evoked myogenic potentials show altered tuning in patients with Meniere's illness. Otol. Neurotol. 25, 333–338 (2004).
-
Kingma, C. 1000. & Wit, H. P. Asymmetric vestibular evoked myogenic potentials in unilateral Meniere patients. Eur. Arch. Otorhinolaryngol. 268, 57–61 (2011).
-
Jariengprasert, C., Ruencharoen, Due south., Reddy, N. 5. & Tiensuwan, M. The sensitivity and specificity of vestibular evoked myogenic potential (VEMP) in the diagnosis of definite Ménière'south affliction patients. J. Otolaryngol. Head Cervix Surg. 3, 009 (2017).
-
McGarvie, L. A., Curthoys, I. S., MacDougall, H. G. & Halmagyi, G. M. What does the caput impulse examination versus caloric dissociation reveal almost vestibular dysfunction in Meniere's disease?. Ann. N. Y. Acad. Sci. 1343, 58–62 (2015).
-
Cureoglu, S., da Costa Monsanto, R. & Paparella, One thousand. M. Histopathology of Meniere's disease. Oper. Tech. Otolayngol. Head Cervix Surg. 27, 194–204 (2016).
-
Leng, Y. & Liu, B. Dissociation of caloric and video head impulse tests in patients with delayed endolymphatic hydrops. Forepart. Neurol. 11, 362 (2020).
-
Eza-Nuñez, P., Guajardo-Vergara, C., Fariñas-Alvarez, C., Arbizu-Ruiz, L. & Pérez-Fernández, North. Dissociated vestibular test results (caloric and vHIT) in patients with Meniere'southward illness are not due to velocity storage malfunction. Hear. Balance Commun. xviii, 136–142 (2020).
-
Naganawa, S. et al. Imaging of Meniere'due south disease after intravenous assistants of unmarried-dose gadodiamide: Utility of subtraction images with different inversion fourth dimension. Magn. Reson. Med. Sci. 11, 213–219 (2012).
-
Schneider, C. A., Rasband, West. S. & Eliceiri, K. Westward. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Acknowledgements
We sincerely appreciate the NIDCD National Temporal Bone, Hearing, and Balance Pathology Resources Registry and the Otopathology Laboratory of Massachusetts Heart and Ear for cooperation and help in completing this paper.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1F1A1073904).
Writer information
Affiliations
Contributions
W.H.C. and Y.South.C. designed the inquiry; Y.S.C., J.South.1000., Grand.B.K., Southward.M.K., and C.H.L. collected the data; Y.S.C., Y.K.K., and H.J.K. analyzed the data; Y.S.C. and W.H.C. wrote the main paper, and West.H.C. provided critical revisions, discussed the results and implications, and commented on the manuscript at all stages.
Corresponding author
Ideals declarations
Competing interests
The authors declare no competing interests.
Additional data
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open up Access This article is licensed under a Creative Commons Attribution iv.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long equally you requite appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the commodity's Creative Commons licence, unless indicated otherwise in a credit line to the cloth. If fabric is non included in the commodity's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, y'all will demand to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Nigh this article
Cite this article
Cho, Y.South., Kim, J.Due south., Kim, Grand.B. et al. Validation of inner ear MRI in patients with Ménière'due south illness by comparison endolymphatic hydrops from histopathologic specimens. Sci Rep 11, 17738 (2021). https://doi.org/x.1038/s41598-021-97213-7
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-021-97213-7
Comments
Past submitting a comment you agree to abide by our Terms and Community Guidelines. If y'all find something calumniating or that does not comply with our terms or guidelines please flag it equally inappropriate.
Source: https://www.nature.com/articles/s41598-021-97213-7
0 Response to "Can an Mri Scan Detect Inner Ear Problems?"
Post a Comment